ectd247 Benefits
- Save time
- Reduce Cost
- Lower compliance risk
- Improve Quality
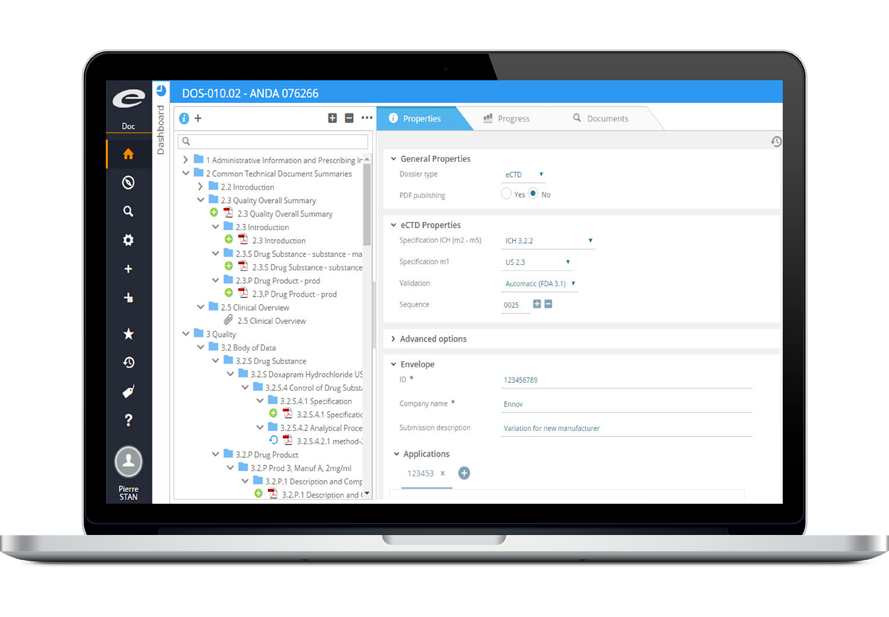
Benefit from a fully integrated electronic process and a completely managed platform.
Simplify
eCTD 247 is a cloud-based fully integrated electronic submissions and document management solution.
With eCTD 247, the eCTD knowhow is embedded into the software itself in a simplified intuitive manner, thus saving you time, money and resources.
Simplified Regulatory Submissions
Prepare, manage and submit your IND, NDA, ANDA or BLA submissions, regardless your level of eCTD expertise.
Compiling an eCTD submission is just the final step. The key is to have all documents ready on time and accessible. eCTD 247 is a cloud platform that helps you securely manage your regulatory content.

Digital

The included document management system (EDMS) enables fast document access through the search engine or by navigation in configurable views. It also manages templates so that users can effortlessly create documents that comply with internal document guidelines. The reporting capabilities also enable to cut audit preparation time.
Original applications, supplements, variations, amendments are fast to build, update and submit thanks to hyperlink and bookmark management, automatic build and edit of Study Tagging Files (STFs), fast access to eCTD status, and drag and drop based dossier construction.

Compliance with 21 CFR Part 11 is facilitated by the native traceability and electronic signature included in eCTD247. eCTD standard compliance can be checked with the integrated eCTD validator. For submissions to European Regulatory Authorities, this validator has also approved by the EMA.
We support all the main existing human & veterinary submission formats, current and older versions (eCTD, NeeS, vNeeS, paper CTD).

eCTD247’s included document management system (EDMS) manages document validation so that all documents follow the pre-defined validation process.
Documents tracked with version control: the EDMS manages document versioning, and documents inserted in dossiers can be automatically refreshed to automatically mirror the latest available document version.
Collaborate
The release of documents is managed so that relevant people are always informed. Authors are also informed when receivers have opened the released documents. Documents can also be reviewed and commented on simultaneously.
Documents and dossiers access rights are also managed so that open or private and public working groups can be created.
Ennov supports parallel work locally and worldwide, providing a full web interface, and managing document check-in / check-out.

Cloud
Infrastructure costs are eliminated (taken care of by Ennov) and required internal IT personnel and services reduced to a minimum. Hosted software updates can occur without requiring work on the sponsor’s part.
Validation activities can also be managed as a part of the cloud offering.
You can login from anywhere in the world and work efficiently with access to all your data. This also provides the ability to grant access to external consultants or partners as needed.
Ennov hosts your data in the United States or in the EU, according to your choice. In both cases, your data and your application are on a highly secure server in a purpsoe built datacentre. User access is fully managed with two-factor authentication, SSL encyption, and a full regulatory compliant audit trail. Data backups are routinely made and our we can ensure a quick recovery in case of disaster.
Updates are performed regularly by Ennov, to improve the software and more importantly to follow the evolution of regulatory standards and regulatory norms. eCTD247 supports current as well as former versions of eCTD, NeeS, vNeeS…


